Colgate explains how dental professionals can use taste to aid adherence in patients with an increased risk of caries.
Dental professionals dedicate time to proactively guide and coach patients in enhancing their oral health through self-care at-home. Employing an evidence-based approach in providing advice and implementing interventions can significantly contribute to achieving superior patient outcomes.
Product efficacy and patient adherence are key factors for optimal clinical outcomes
A prescribed toothpaste can be a factor in patients’ daily adherence to at-home oral care and may act as a barrier to change of behaviour and habits. Experiencing displeasure when brushing could affect dose and duration of use, taste being an influential factor. This means recommending a product with a preferred taste over another can benefit patient adherence.
Taste has an influential role in adhering to toothpaste
Colgate performed a single blinded, non-interventional study (Colgate, 2020). Participants were asked to taste and compare Duraphat 5000 ppm fluoride toothpaste with another generic 5000 ppm fluoride toothpaste.
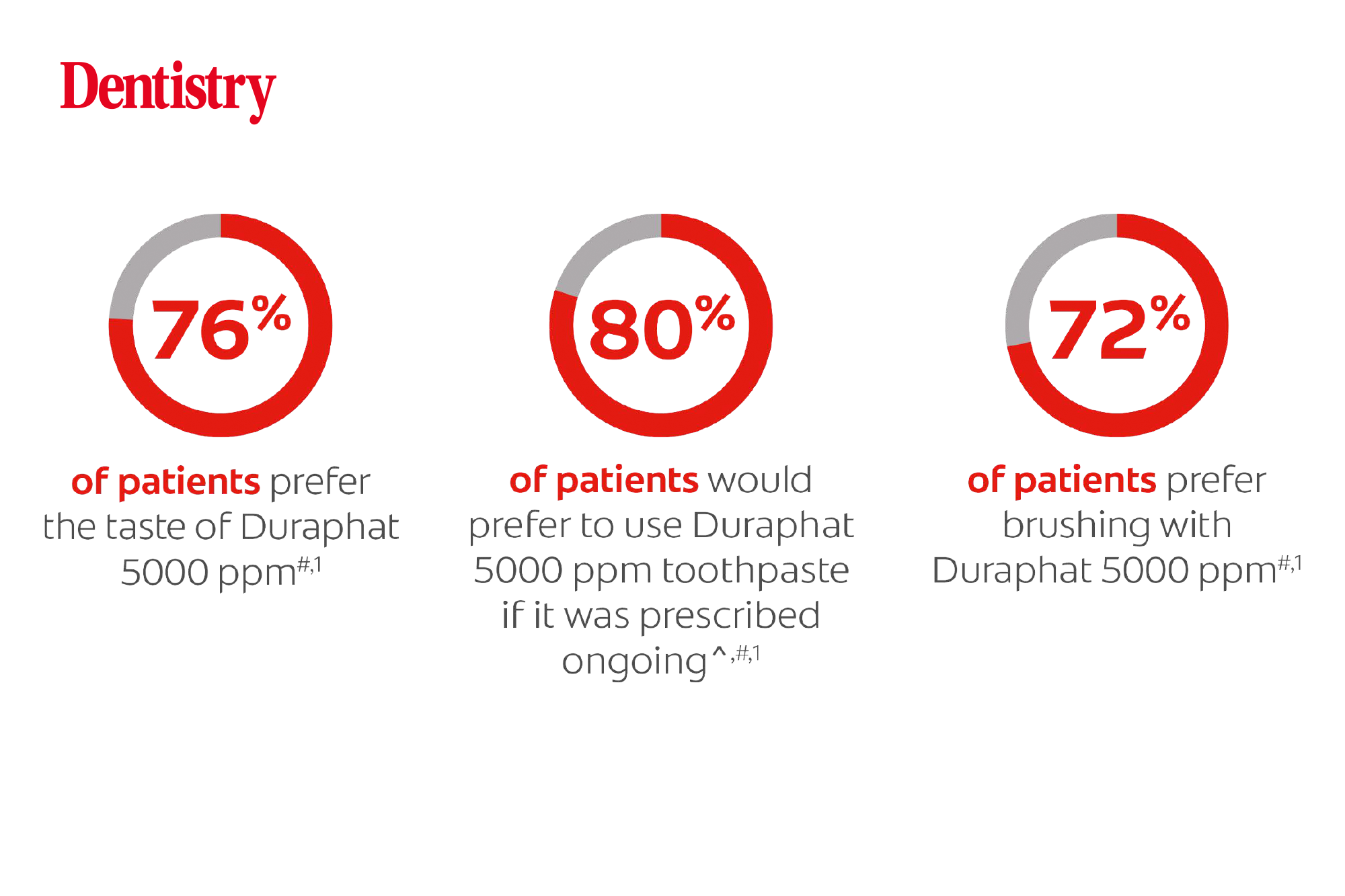
Results demonstrated the importance of taste preference when considering which toothpaste to prescribe patients at increased risk of dental caries. Nearly three quarters of those surveyed preferred the taste of the Duraphat to the studied leading generic paste, with a similar level saying they found that using Duraphat was preferable brushing experience.
Interestingly, the patient questionnaires also highlighted that 80% of those surveyed would prefer Colgate Duraphat if it was prescribed ongoing.
- 76% of patients prefer the taste of Durophat 5000 ppm (Colgate, 2020)*
- 80% of patients would prefer to use Durophat 5000 ppm if it was prescribed ongoing (Colgate, 2020)* ****
- 72% of patients prefer brushing with Durophat 5000ppm (Colgate, 2020).*
Support patients with increased risk of caries by prescribing the brand they will prefer***
Colgate Duraphat 5000 ppm Fluoride Toothpaste is clinically proven to prevent cavities by arresting and reversing primary root and early fissure caries lesion (Baysan et al, 2001, Schirrmeister et al, 2007, Ekstrand et al, 2008, Ekstrand et al, 2013). Prescribe by the brand name if you would like your patient to receive Colgate Duraphat 5000 ppm Fluoride Toothpaste.
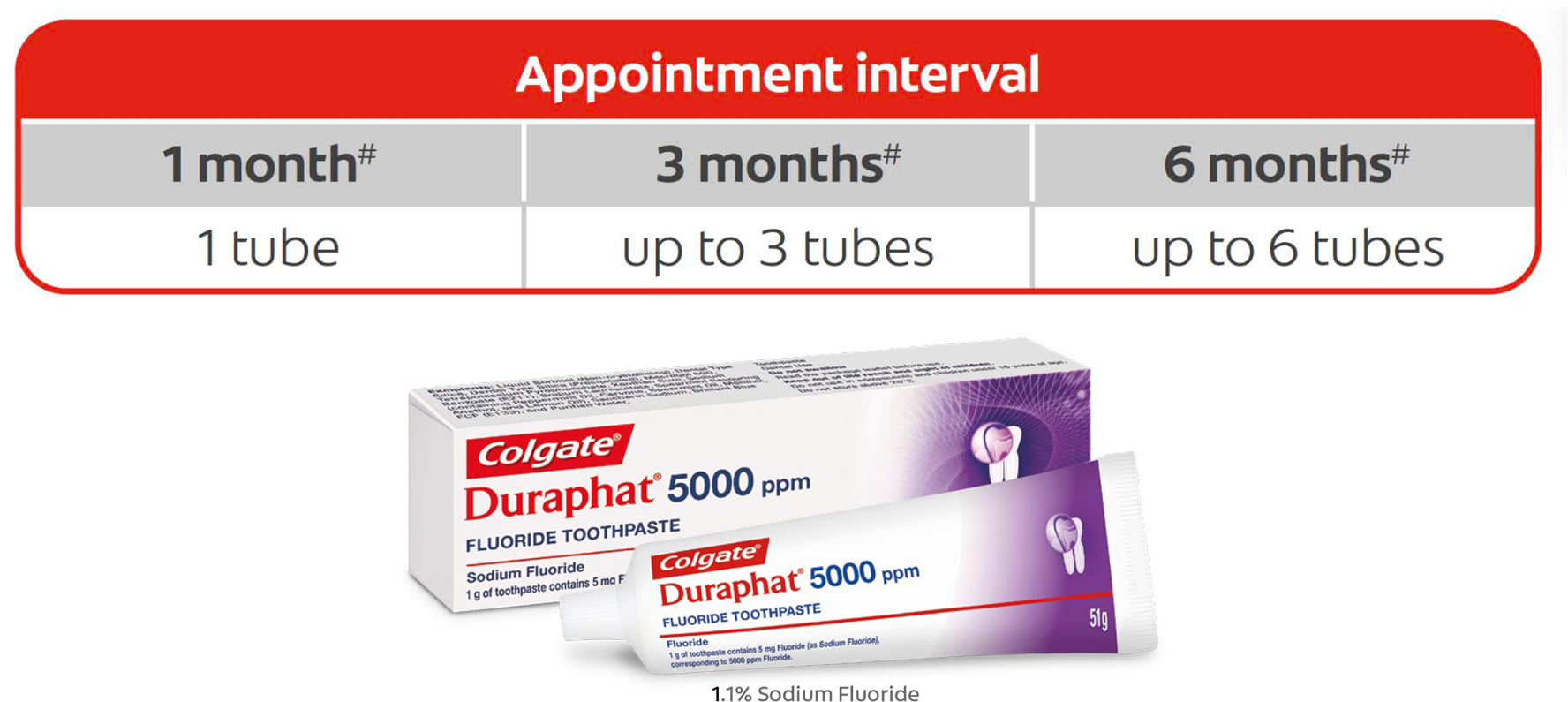
Course of treatment
Clinical evidence for Colgate Duraphat 5000 ppm Flouride Toothpaste supports its continued use to optimise the prevention of caries (Baysan et al, 2001, Schirrmeister et al, 2007, Ekstrand et al, 2008, Ekstrand et al, 2013). Consider the date of the patient’s next recall appointment and prescribe enough tubes to last until then.
Prescribe by brand to ensure your patients receive Colgate Duraphat
For more information, visit www.colgateprofessional.com.hk/products/products-list/colgate-5000-duraphat.
* Compared to leading generic 5000 ppm high fluoride toothpaste. ** Patients ≥ 16 years at increased caries risk. *** Colgate UK Consumer Survey on Cosmetic Toothpaste. 504 participants. Feb 2020. **** for four weeks or more. †† Usage as according to the summary of product characteristics.
References
1. Data on file. Preference Survey. January 2020 (n=82).
2. Baysan A et al. Caries Res 2001;35:41-46.
3. Schirrmeister JF et al. Am J Dent 2007;20. 212-216.
4. Ekstrand et al. Geodent 2008;25:67-75.
5. Ekstrand et al. Caries Res 2013;47:391–8.
Prescribing information
Name of the medicinal product: Duraphat 5000 ppm Fluoride Toothpaste.
Active ingredient: Sodium Fluoride 1.1%w/w (5000ppm F-). 1g of toothpaste contains 5mg fluoride (as sodium fluoride), corresponding to 5000ppm fluoride.
Indications: For the prevention of dental caries in adolescents and adults over 16 years of age, particularly amongst patients at risk from multiple caries (coronal and/or root caries).
Dosage and administration: Brush carefully on a daily basis applying a 2cm ribbon onto the toothbrush for each brushing. Three times daily, after each meal.
Contraindications: This medicinal product must not be used in cases of hypersensitivity to the active substance or to any of the excipients.
Special warnings and precautions for use: An increased number of potential fluoride sources may lead to fluorosis. Before using fluoride medicines such as Duraphat, an assessment of overall fluoride intake (ie drinking water, fluoridated salt, other fluoride medicines – tablets, drops, gum or toothpaste) should be done. Fluoride tablets, drops, chewing gum, gels or varnishes and fluoridated water or salt should be avoided during use of Duraphat Toothpaste. When carrying out overall calculations of the recommended fluoride ion intake, which is 0.05mg/kg per day from all sources, not exceeding 1mg per day, allowance must be made for possible ingestion of toothpaste (each tube of Duraphat 500mg/100g Toothpaste contains 255mg of fluoride ions). Contains Sodium Benzoate. Sodium Benzoate which may cause local irritation. Contains a spearmint flavouring with allergens (limonene, linalool, citral, geraniol, and citronellol) which may cause allergic reactions.
Undesirable effects:
Gastrointestinal disorders: frequency not known (cannot be estimated from the available data): Burning oral sensation. Immune system disorders: Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions.
Legal classification: POM.
Marketing authorisation number: PL00049/0050.
Marketing authorisation holder: Colgate-Palmolive (U.K.) Ltd. Guildford Business Park, Midleton Road, Guildford, Surrey, GU2 8JZ.
Recommended retail price: £7.99 (51g tube). Date of revision of text: July 2022.


